Analysis of PBMC4k using Seurat
Introduction
library(Seurat)
library(gridExtra)
Loading the raw UMI count matrix
First we load the raw UMI matrix into the R environment. Seurat provides a Read10X function to import a sparse matrix generated by cellranger count.
mat.raw <- Read10X(data.dir = "output_cellranger_full/outs/filtered_gene_bc_matrices/GRCh38")
dim(mat.raw)
## [1] 33694 4340
We start here with the pre-filtered matrix provided by cellranger containing the set of 4321 high-confidence cells. So we can proceed immediately to create the Seurat object.
sobj <- CreateSeuratObject(raw.data=mat.raw, min.cells = 5)
dim(sobj@data)
## [1] 15411 4340
Downstream analysis
Unlike in the previous tutorial, here the commands and results for each section are hidden by default. Try to go through each exercise on your own, by adapting the commands we used in MCA tutorial and running them in the R console.
When you arrive at a solution, or if you get stuck, click on the solution link below each section to show one possible approach to the problem.
Exercise 1: Filter the raw UMI matrix
Check the distributions of UMI, gene counts and percent of mitochondrial RNA, and filter the barcodes appropriately.
Note: while in the mouse genome annotation mitochondrial genes have names that start with mt- (lowercase), in the human annotation mitochondrial genes start with MT- (uppercase).
Click Here to see one solution
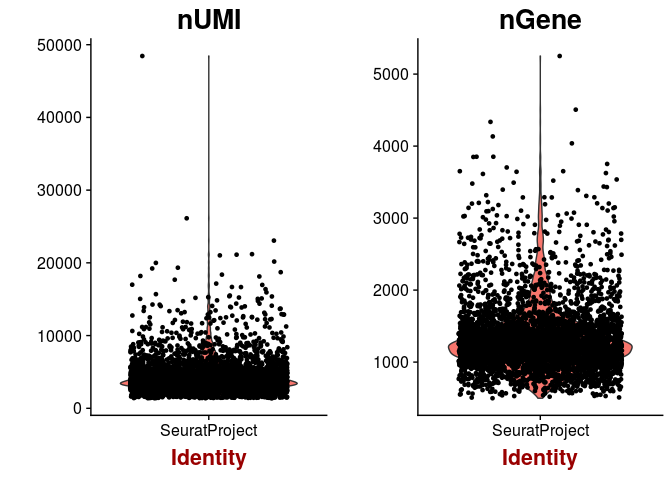
VlnPlot(sobj, features.plot = c("nUMI", "nGene"))
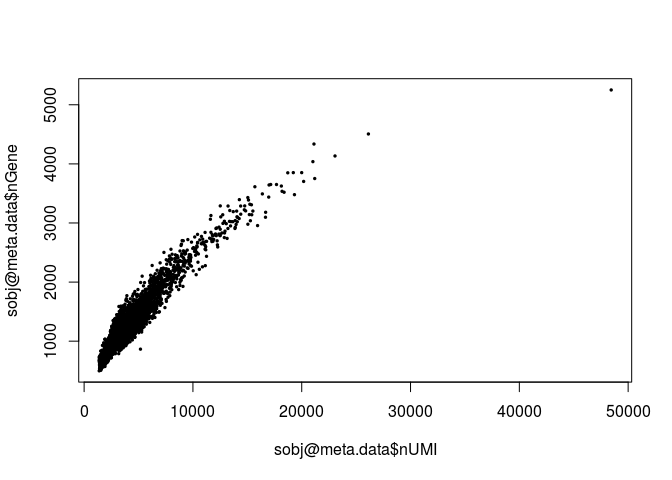
plot(sobj@meta.data$nUMI, sobj@meta.data$nGene, pch=20, cex=0.5)
mito.genes <- grep("^MT-", rownames(sobj@data), value = TRUE)
percent.mito <- Matrix::colSums(sobj@data[mito.genes, ]) / Matrix::colSums(sobj@data)
sobj <- AddMetaData(sobj, metadata = percent.mito, col.name = "percent.mito")
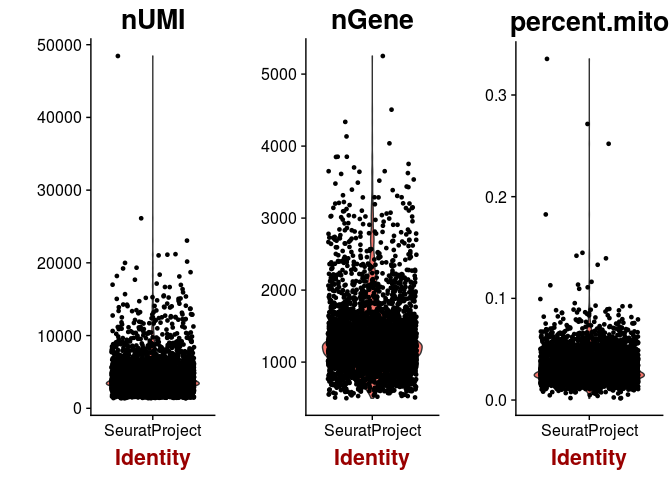
VlnPlot(sobj, features.plot = c("nUMI", "nGene", "percent.mito"))
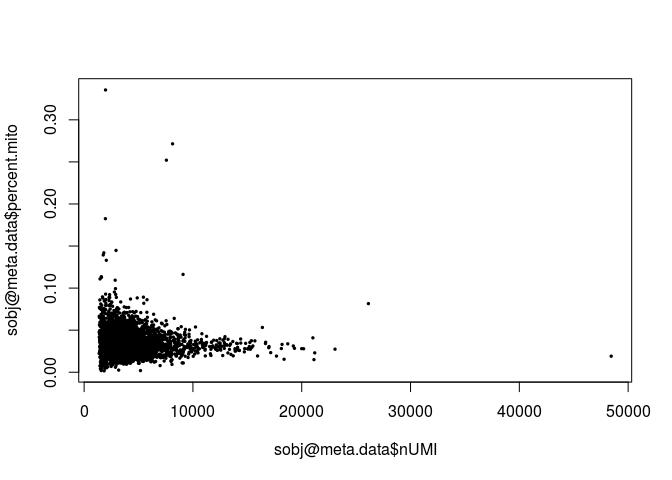
plot(sobj@meta.data$nUMI, sobj@meta.data$percent.mito, pch=20, cex=0.5)
sobj <- FilterCells(sobj, subset.names = "nGene", high.thresholds = 3000) sobj <- FilterCells(sobj, subset.names = "percent.mito", high.thresholds = 0.1) dim(sobj@data)
## [1] 15411 4272
Exercise 2: Normalize the raw counts
Click Here to see one solution
sobj <- NormalizeData(sobj, normalization.method = "LogNormalize", scale.factor = median(sobj@meta.data$nUMI))
Exercise 3: Select a subset of highly variable genes to use for dimensionality reduction and clustering
Hint: Aim for a subset of 1,000 to 2,000 genes.
Click Here to see one solution
sobj <- FindVariableGenes(sobj, mean.function = ExpMean, dispersion.function = LogVMR,
x.low.cutoff = 0.0125, x.high.cutoff = 3, y.cutoff = 0.5)
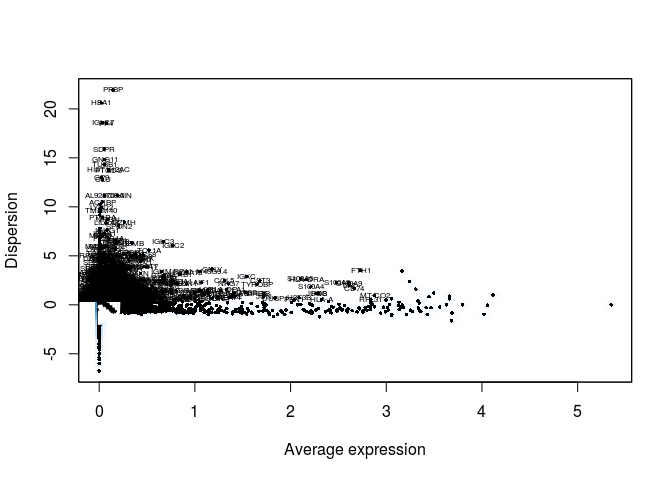
length(sobj@var.genes)
## [1] 1252
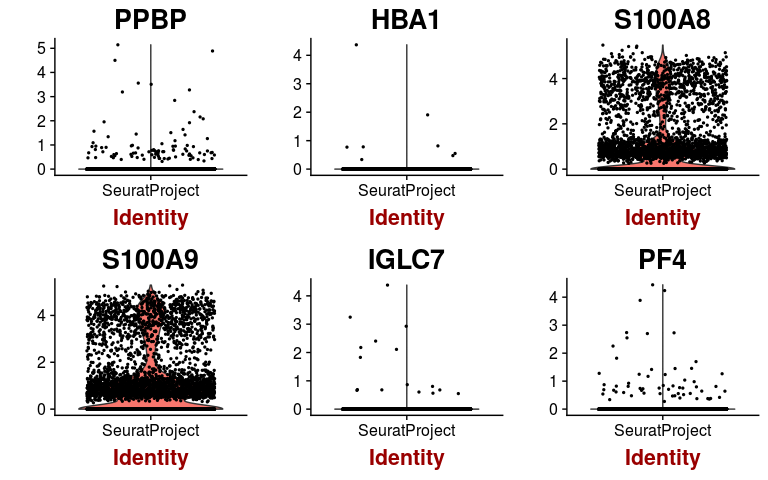
hvginfo <- sobj@hvg.info[ sobj@var.genes, ] highest.dispersion <- head(rownames(hvginfo)[ order(-hvginfo$gene.dispersion) ]) highest.mean <- head(rownames(hvginfo)[ order(-hvginfo$gene.mean) ]) VlnPlot(sobj, features.plot = highest.dispersion, point.size.use=0.5)
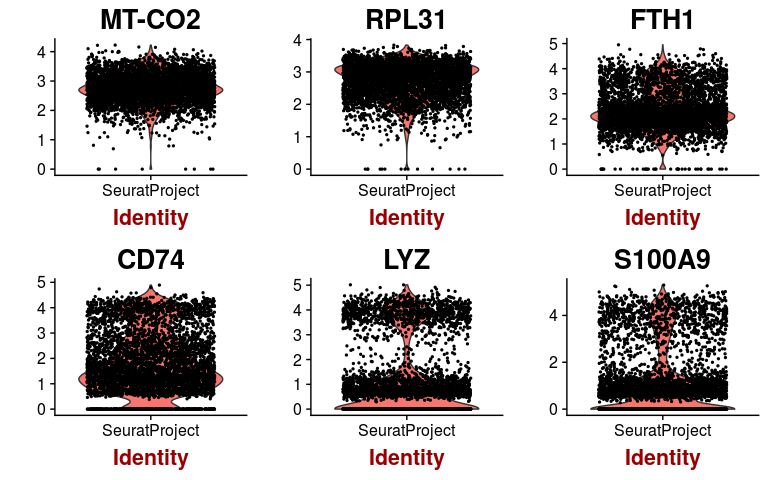
VlnPlot(sobj, features.plot = highest.mean, point.size.use=0.5)
Exercise 4: Scale the normalized matrix, and perform a principal component analysis (PCA)
After obtaining and visualizing the PCA, determine the number of PCs you are going to use for further analysis.
Click Here to see one solution
sobj <- ScaleData(object = sobj, vars.to.regress = c("nUMI", "percent.mito"))
## Regressing out: nUMI, percent.mito
## ## Time Elapsed: 21.6098577976227 secs
## Scaling data matrix
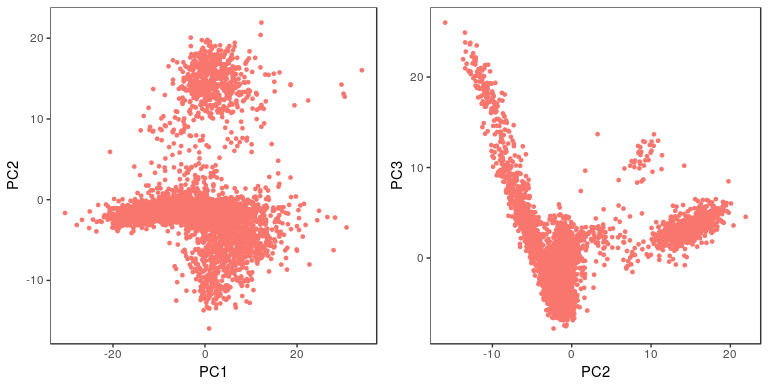
sobj <- RunPCA(object = sobj, pc.genes = sobj@var.genes, pcs.compute = 40, do.print=FALSE) p1 <- PCAPlot(object = sobj, dim.1 = 1, dim.2 = 2, do.return=TRUE) + theme(legend.pos="none") p2 <- PCAPlot(object = sobj, dim.1 = 2, dim.2 = 3, do.return=TRUE) + theme(legend.pos="none") grid.arrange(p1, p2, ncol=2)
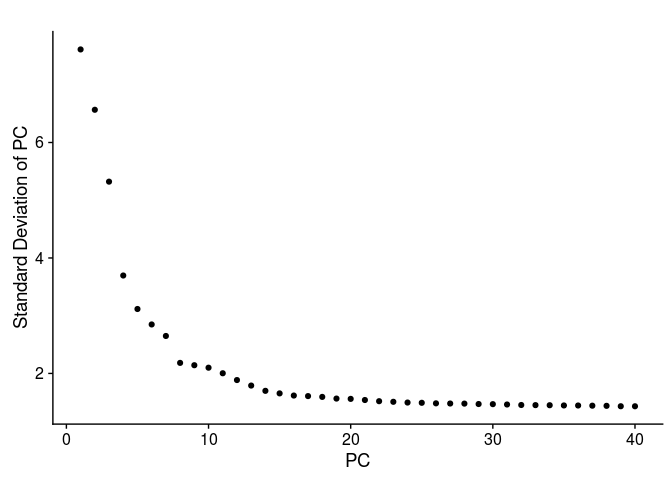
PCElbowPlot(sobj, num.pc = 40)
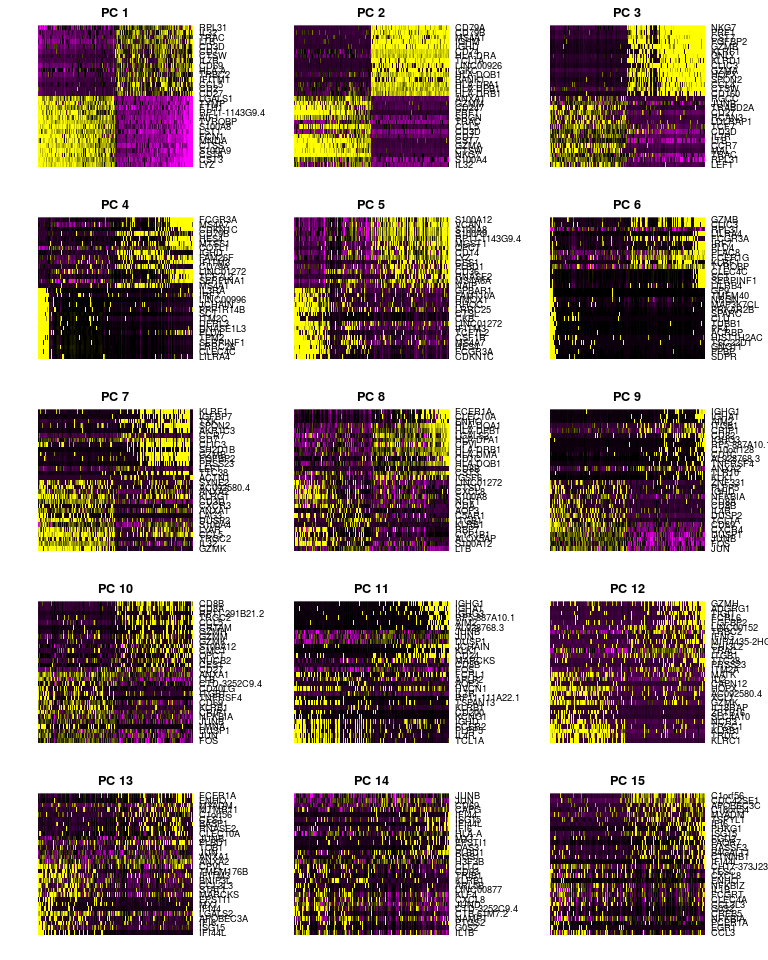
PCHeatmap(sobj, pc.use = 1:15, cells.use = 500, do.balanced = TRUE, label.columns = FALSE)
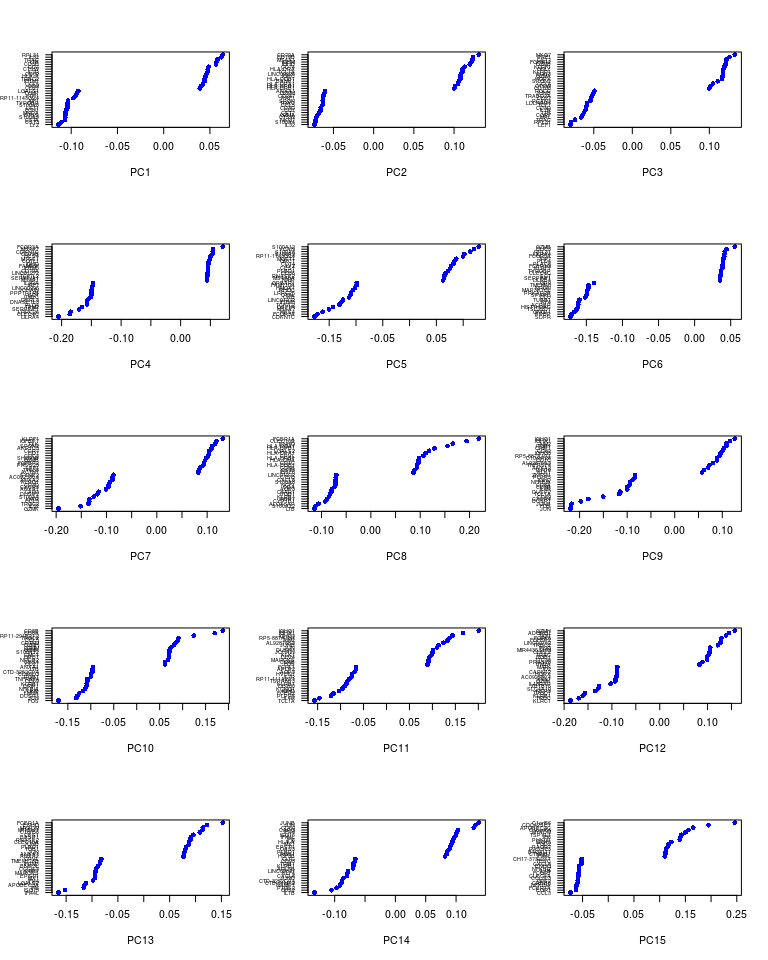
VizPCA(sobj, pcs.use = 1:15, do.balanced = TRUE)
# sobj <- JackStraw(sobj, num.pc = 40, num.replicate = 50, do.par=TRUE, display.progress = FALSE) # sobj <- JackStrawPlot(sobj, PCs = 1:40) # # plot(1:40, -log10(sobj@dr$pca@jackstraw@overall.p.values[,2])) # abline(h=-log10(0.05))
Exercise 5: Cluster the cells in the expression matrix and represent the clusters in a t-SNE plot
Try to select an appropriate value for the clustering resolution parameter, and the perplexity parameter of the t-SNE.
Click Here to see one solution
sobj <- FindClusters(sobj, reduction.type = "pca", dims.use = 1:15,
resolution = 0.8, print.output = 0, save.SNN = FALSE)
sobj <- RunTSNE(sobj, dims.use = 1:15, do.fast = TRUE, perplexity = 30)
TSNEPlot(sobj, do.label = TRUE)
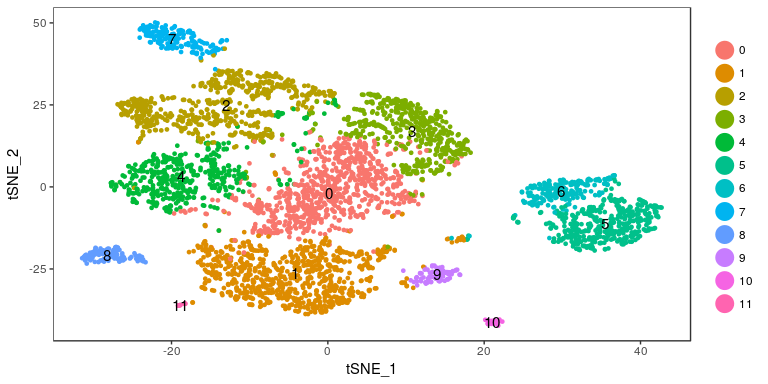
Exercise 6: Find marker genes for all clusters
Examine the top markers for all clusters, and determine if any of the clusters seem to be very similar. If this is the case, group similar clusters together, and repeat the marker gene discovery on the new clusters.
Click Here to see one solution
markers <- FindAllMarkers(object = sobj, only.pos = TRUE, min.pct = 0.25, thresh.use = 0.25) markers <- markers[ markers$p_val_adj < 0.01, ] head(markers)
## p_val avg_logFC pct.1 pct.2 p_val_adj cluster gene ## RPL21 9.595436e-177 0.3426853 1.000 0.999 1.478753e-172 0 RPL21 ## RPS27 5.806036e-170 0.3684605 1.000 1.000 8.947683e-166 0 RPS27 ## RPL31 5.196314e-163 0.4276901 0.998 0.997 8.008040e-159 0 RPL31 ## RPL32 1.498597e-162 0.3333247 1.000 1.000 2.309487e-158 0 RPL32 ## RPL34 1.495631e-161 0.3295687 1.000 0.999 2.304916e-157 0 RPL34 ## RPS14 3.348956e-158 0.3324048 1.000 1.000 5.161076e-154 0 RPS14
top.markers <- do.call(rbind, lapply(split(markers, markers$cluster), head)) DoHeatmap(sobj, genes.use = top.markers$gene, slim.col.label = TRUE, remove.key = TRUE)
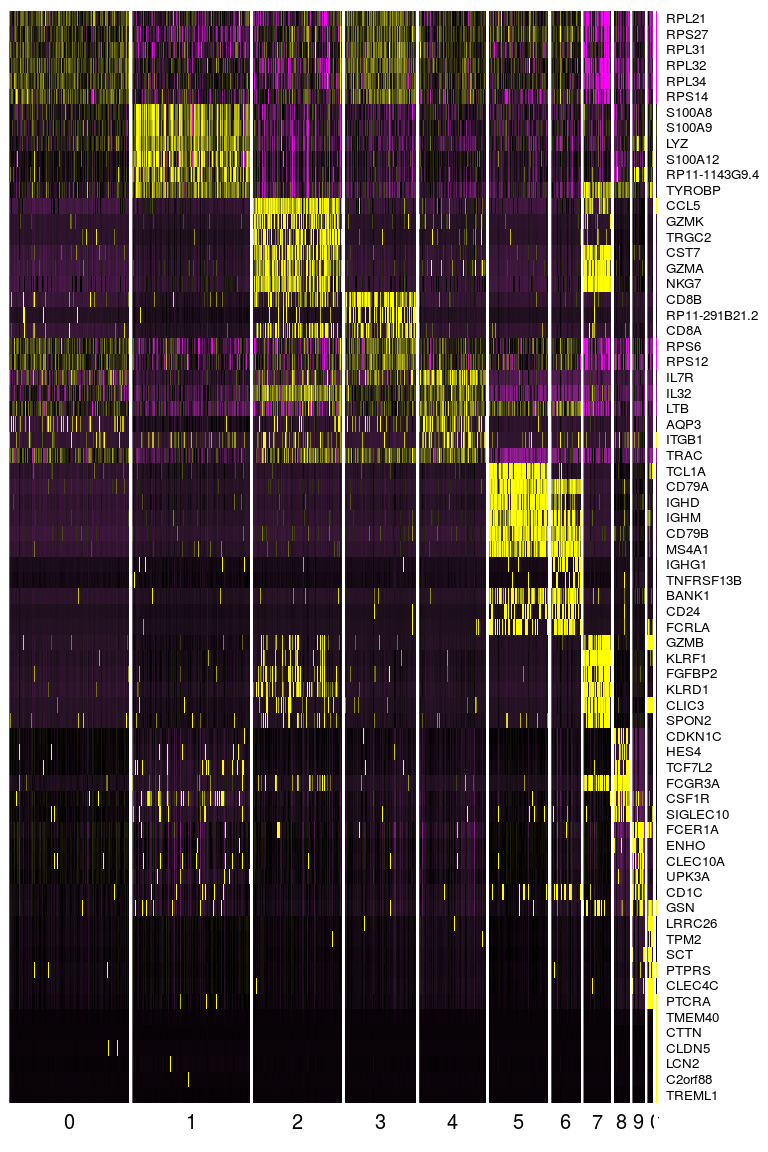
Exercise 7: Identify cell subpolulations
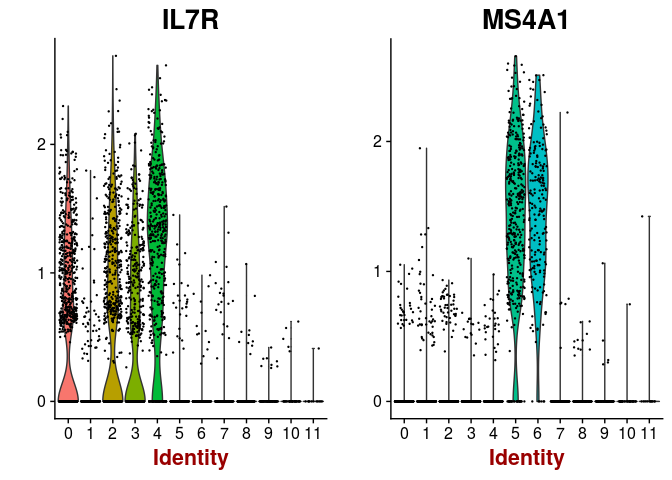
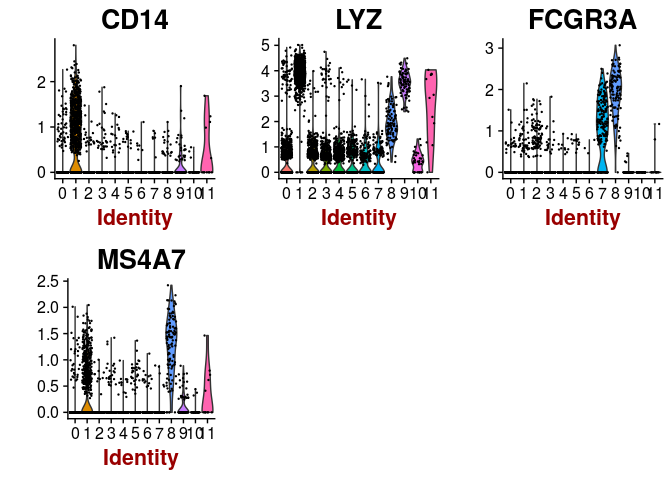
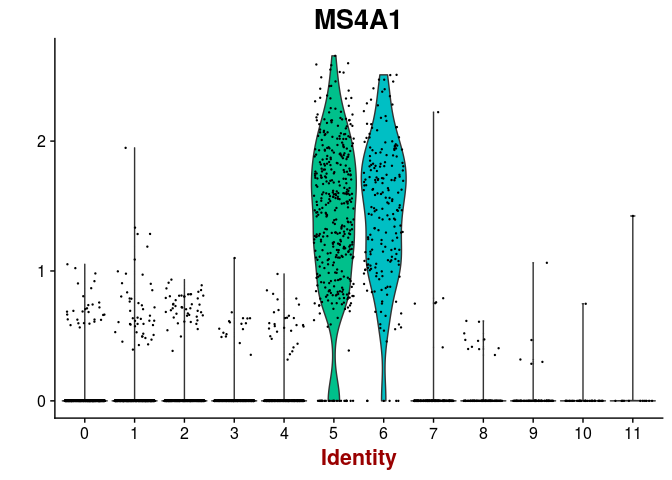
Use the VlnPlot and FeaturePlot functions to examine the expresssion of the marker genes below. Can you indentify some of the cell subpopulations?
| Markers | Cell Type |
|---|---|
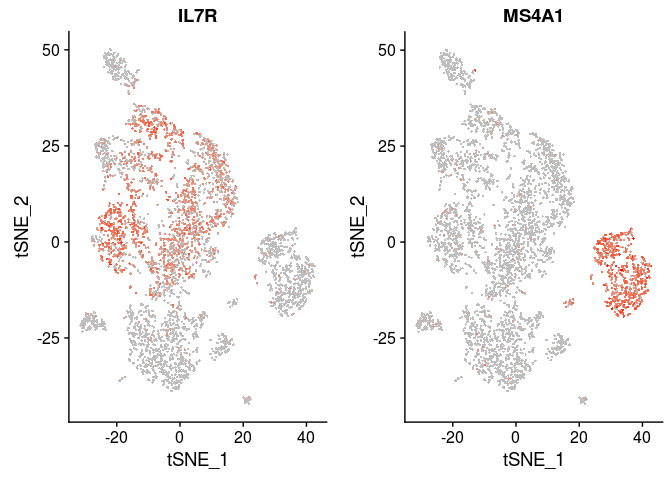
| IL7R | CD4 T cells |
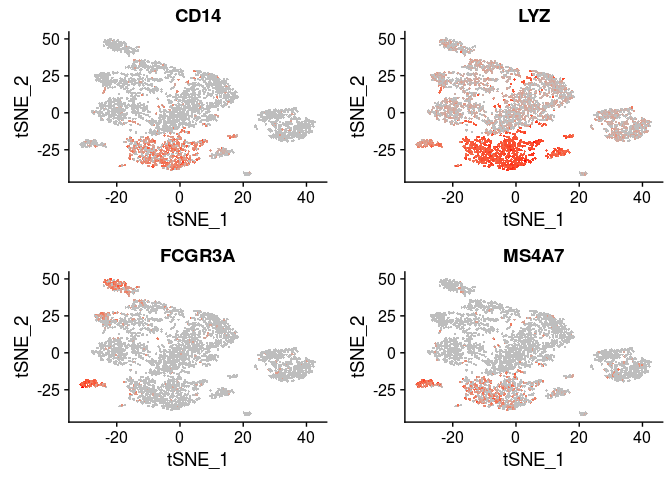
| CD14, LYZ | CD14+ Monocytes |
| MS4A1 | B cells |
| CD8A | CD8 T cells |
| FCGR3A, MS4A7 FCGR3A+ | Monocytes |
| GNLY, NKG7 | NK cells |
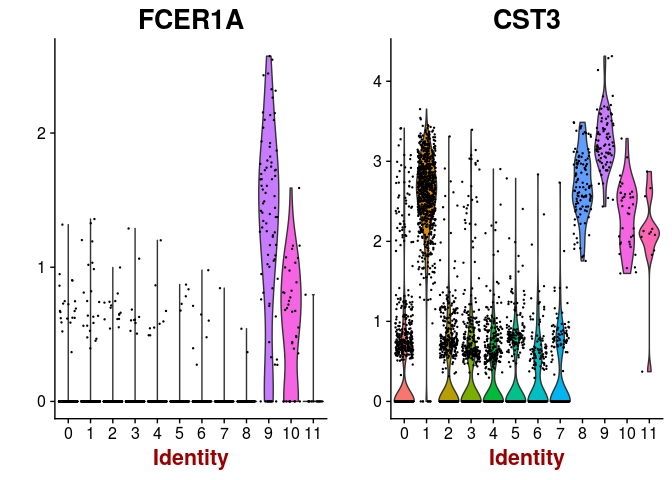
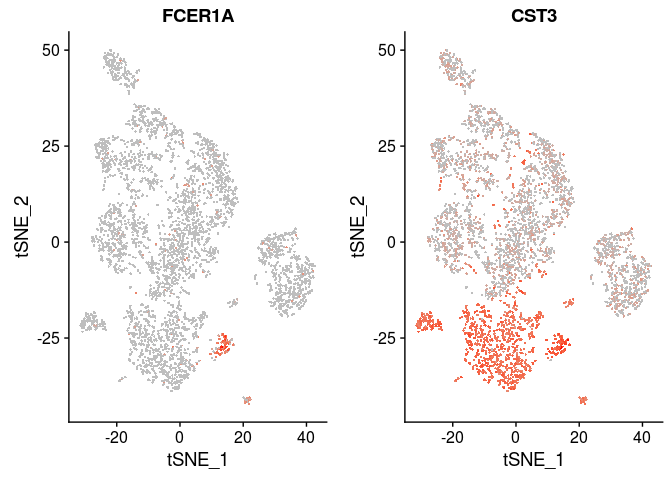
| FCER1A, CST3 | Dendritic Cells |
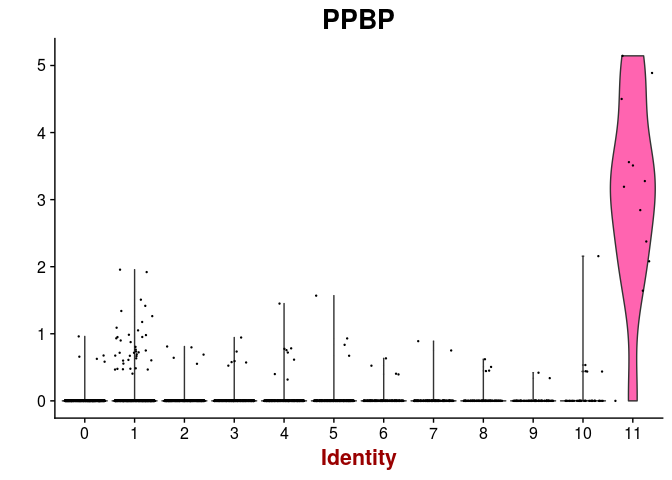
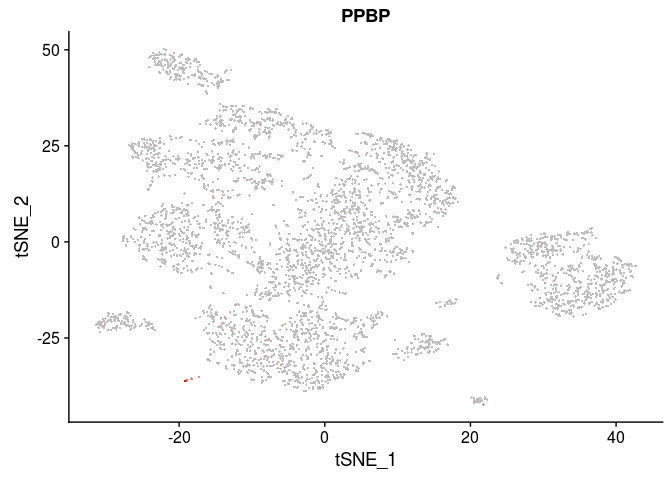
| PPBP | Megakaryocytes |
Click Here to see one solution
VlnPlot(sobj, features.plot = c("IL7R", "MS4A1"), point.size.use=0.2)
VlnPlot(sobj, features.plot = c("CD14", "LYZ", "FCGR3A", "MS4A7"), point.size.use=0.2)
VlnPlot(sobj, features.plot = c("MS4A1"), point.size.use=0.2)
VlnPlot(sobj, features.plot = c("FCER1A", "CST3"), point.size.use=0.2)
VlnPlot(sobj, features.plot = c("PPBP"), point.size.use=0.2)
FeaturePlot(sobj, features.plot = c("IL7R", "MS4A1"), cols.use=c("grey", "red"), pt.size=0.5)
FeaturePlot(sobj, features.plot = c("CD14", "LYZ", "FCGR3A", "MS4A7"), cols.use=c("grey", "red"), pt.size=0.5)
FeaturePlot(sobj, features.plot = c("MS4A1"), cols.use=c("grey", "red"), pt.size=0.5)
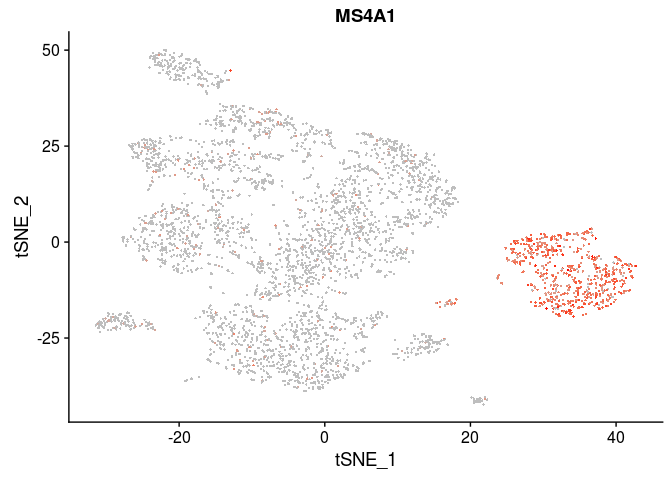
FeaturePlot(sobj, features.plot = c("FCER1A", "CST3"), cols.use=c("grey", "red"), pt.size=0.5)
FeaturePlot(sobj, features.plot = c("PPBP"), cols.use=c("grey", "red"), pt.size=0.5)
Session Information
sessionInfo()
## R version 3.4.4 (2018-03-15)
## Platform: x86_64-pc-linux-gnu (64-bit)
## Running under: Ubuntu 16.04.4 LTS
##
## Matrix products: default
## BLAS: /usr/lib/libblas/libblas.so.3.6.0
## LAPACK: /usr/lib/lapack/liblapack.so.3.6.0
##
## locale:
## [1] LC_CTYPE=pt_PT.UTF-8 LC_NUMERIC=C
## [3] LC_TIME=pt_PT.UTF-8 LC_COLLATE=en_US.UTF-8
## [5] LC_MONETARY=pt_PT.UTF-8 LC_MESSAGES=en_US.UTF-8
## [7] LC_PAPER=pt_PT.UTF-8 LC_NAME=C
## [9] LC_ADDRESS=C LC_TELEPHONE=C
## [11] LC_MEASUREMENT=pt_PT.UTF-8 LC_IDENTIFICATION=C
##
## attached base packages:
## [1] stats graphics grDevices utils datasets methods base
##
## other attached packages:
## [1] bindrcpp_0.2.2 gridExtra_2.3 Seurat_2.3.3 Matrix_1.2-14
## [5] cowplot_0.9.2 ggplot2_2.2.1
##
## loaded via a namespace (and not attached):
## [1] tsne_0.1-3 segmented_0.5-3.0 nlme_3.1-137
## [4] bitops_1.0-6 bit64_0.9-7 RColorBrewer_1.1-2
## [7] rprojroot_1.3-2 prabclus_2.2-6 tools_3.4.4
## [10] backports_1.1.2 irlba_2.3.2 R6_2.2.2
## [13] rpart_4.1-13 KernSmooth_2.23-15 Hmisc_4.1-1
## [16] lazyeval_0.2.1 colorspace_1.3-2 trimcluster_0.1-2
## [19] nnet_7.3-12 tidyselect_0.2.4 diffusionMap_1.1-0
## [22] bit_1.1-12 compiler_3.4.4 htmlTable_1.11.1
## [25] hdf5r_1.0.0 labeling_0.3 diptest_0.75-7
## [28] caTools_1.17.1 scales_1.0.0 checkmate_1.8.5
## [31] lmtest_0.9-35 DEoptimR_1.0-8 mvtnorm_1.0-6
## [34] robustbase_0.92-8 ggridges_0.5.0 pbapply_1.3-4
## [37] dtw_1.18-1 proxy_0.4-21 stringr_1.3.1
## [40] digest_0.6.16 mixtools_1.1.0 foreign_0.8-70
## [43] rmarkdown_1.9 R.utils_2.6.0 base64enc_0.1-3
## [46] pkgconfig_2.0.2 htmltools_0.3.6 htmlwidgets_1.2
## [49] rlang_0.2.2 rstudioapi_0.7 bindr_0.1.1
## [52] jsonlite_1.5 zoo_1.8-1 ica_1.0-1
## [55] mclust_5.4 gtools_3.5.0 acepack_1.4.1
## [58] dplyr_0.7.6 R.oo_1.21.0 magrittr_1.5
## [61] modeltools_0.2-21 Formula_1.2-2 lars_1.2
## [64] Rcpp_0.12.18 munsell_0.5.0 reticulate_1.5
## [67] ape_5.1 R.methodsS3_1.7.1 scatterplot3d_0.3-40
## [70] stringi_1.2.4 yaml_2.2.0 MASS_7.3-50
## [73] flexmix_2.3-13 gplots_3.0.1 Rtsne_0.13
## [76] plyr_1.8.4 grid_3.4.4 parallel_3.4.4
## [79] gdata_2.18.0 crayon_1.3.4 doSNOW_1.0.16
## [82] lattice_0.20-35 splines_3.4.4 SDMTools_1.1-221
## [85] knitr_1.20 pillar_1.3.0 igraph_1.2.1
## [88] fpc_2.1-10 reshape2_1.4.3 codetools_0.2-15
## [91] stats4_3.4.4 glue_1.3.0 evaluate_0.10.1
## [94] metap_0.9 latticeExtra_0.6-28 data.table_1.11.4
## [97] png_0.1-7 foreach_1.4.4 tidyr_0.8.1
## [100] gtable_0.2.0 RANN_2.5.1 purrr_0.2.5
## [103] kernlab_0.9-25 assertthat_0.2.0 class_7.3-14
## [106] survival_2.42-3 tibble_1.4.2 snow_0.4-2
## [109] iterators_1.0.9 cluster_2.0.6 fitdistrplus_1.0-9
## [112] ROCR_1.0-7
References
Back
Back to previous page.