Exercise 1 - Using DESeq2 in R
This document demonstrates how to use DESeq2 in the R environment to perform a differential expression analysis using the the Trapnell datasets as an example. We will first need to tell R what samples are going to be analysed, then run the DESeq2 pipeline and plot the results of the analysis.
Setting up the environment
First we need to make sure that R is running on the same directory where we placed the counts files (the files called trapnell_counts_C1_R1.tab, trapnell_counts_C1_R2.tab, etc…). To do this either type setwd("path/to/directory") in the R console, or use the Files panel to navigate to the counts directory and then select More -> Set As Working Directory.
Setting up the count data and metadata
In this example, instead of loading the sample counts ourselves, we are going to let DESeq2 handle that for us. For this, we just need to tell DESeq2 what files correspond to each sample. We start by setting variables to hold the list of samples we are going to analyze. We create a list of sample names, a list of sample files (where the counts are), and a list of experimental conditions, telling which samples correspond to each condition. Type the following lines in the R console and press Enter.
sampleNames <- c("trapnell_counts_C1_R1", "trapnell_counts_C1_R2", "trapnell_counts_C1_R3", "trapnell_counts_C2_R1", "trapnell_counts_C2_R2", "trapnell_counts_C2_R3")
sampleFiles <- c("trapnell_counts_C1_R1.tab", "trapnell_counts_C1_R2.tab", "trapnell_counts_C1_R3.tab", "trapnell_counts_C2_R1.tab", "trapnell_counts_C2_R2.tab", "trapnell_counts_C2_R3.tab")
sampleConditions <- c("C1", "C1", "C1", "C2", "C2", "C2")
We can confirm the values in these variables by simply typing a variable name in the R console and pressing Enter.
sampleNames
## [1] "trapnell_counts_C1_R1" "trapnell_counts_C1_R2" "trapnell_counts_C1_R3"
## [4] "trapnell_counts_C2_R1" "trapnell_counts_C2_R2" "trapnell_counts_C2_R3"
sampleFiles
## [1] "trapnell_counts_C1_R1.tab" "trapnell_counts_C1_R2.tab"
## [3] "trapnell_counts_C1_R3.tab" "trapnell_counts_C2_R1.tab"
## [5] "trapnell_counts_C2_R2.tab" "trapnell_counts_C2_R3.tab"
sampleConditions
## [1] "C1" "C1" "C1" "C2" "C2" "C2"
For convenience, we place this information in a table variable that we call sampleTable.
sampleTable <- data.frame(sampleName = sampleNames,
fileName = sampleFiles,
condition = sampleConditions)
sampleTable
## sampleName fileName condition
## 1 trapnell_counts_C1_R1 trapnell_counts_C1_R1.tab C1
## 2 trapnell_counts_C1_R2 trapnell_counts_C1_R2.tab C1
## 3 trapnell_counts_C1_R3 trapnell_counts_C1_R3.tab C1
## 4 trapnell_counts_C2_R1 trapnell_counts_C2_R1.tab C2
## 5 trapnell_counts_C2_R2 trapnell_counts_C2_R2.tab C2
## 6 trapnell_counts_C2_R3 trapnell_counts_C2_R3.tab C2
Running a differential expression test with DESeq2
With the sample table prepared, we are ready to run DESeq2. First need to import it into the R environment. This is done with the library command.
library("DESeq2")
Then, we prepare a special structure to tell DESeq2 what samples we are going to analyse (our sample table), and what comparison we are goind to make. By setting the design argument to ~ condition, we are specifying that column as the experimental variable (C1 or C2).
ddsHTSeq <- DESeqDataSetFromHTSeqCount(sampleTable = sampleTable,
design= ~ condition)
We can run the whole DESeq2 pipeline with a single command. This will perform normalization of the raw counts, estimate variances, and perform the differential expression tests.
ddsHTSeq <- DESeq(ddsHTSeq)
## estimating size factors
## estimating dispersions
## gene-wise dispersion estimates
## mean-dispersion relationship
## final dispersion estimates
## fitting model and testing
We can then extract the results of the differential expression in the form of a table using the results function. The head function will print the first lines of this table on the console.
resHTSeq <- results(ddsHTSeq)
head(resHTSeq)
## log2 fold change (MLE): condition C2 vs C1
## Wald test p-value: condition C2 vs C1
## DataFrame with 6 rows and 6 columns
## baseMean log2FoldChange lfcSE stat pvalue
## <numeric> <numeric> <numeric> <numeric> <numeric>
## FBgn0000003 0.00000 NA NA NA NA
## FBgn0000008 578.61667 -0.01363018 0.09778363 -0.1393912 0.88914103
## FBgn0000014 98.25848 0.26279172 0.22066004 1.1909348 0.23367917
## FBgn0000015 65.03895 0.12821306 0.26373025 0.4861523 0.62685920
## FBgn0000017 2539.89350 0.11317799 0.05904858 1.9166927 0.05527698
## FBgn0000018 327.26607 -0.06538546 0.12276560 -0.5326041 0.59430768
## padj
## <numeric>
## FBgn0000003 NA
## FBgn0000008 0.9992327
## FBgn0000014 0.9515670
## FBgn0000015 NA
## FBgn0000017 0.6446372
## FBgn0000018 0.9992327
Hint: you can type View(resHTSeq) to open the full table in a separate window
We can ask how many genes are differentially expressed (using a cutoff of 0.05) with this command.
table(resHTSeq$padj < 0.05)
##
## FALSE TRUE
## 6322 267
Question: How many genes have padj less than 0.01? How many genes have nominal p-values less than 0.01?
Click Here to see the answer
table(resHTSeq$padj < 0.01)
## ## FALSE TRUE ## 6330 259
table(resHTSeq$pvalue < 0.01)
## ## FALSE TRUE ## 9893 315
We sort this table by p-value (smaller p-values on top), and save it to a file so that we can later import it into Excel.
orderedRes <- resHTSeq[ order(resHTSeq$padj), ]
write.csv(as.data.frame(orderedRes), file="trapnell_C1_VS_C2.DESeq2.csv")
We can also retrieve and save a table of normalized counts.
normCounts <- counts(ddsHTSeq, normalized = TRUE)
head(normCounts)
## trapnell_counts_C1_R1 trapnell_counts_C1_R2
## FBgn0000003 0.00000 0.00000
## FBgn0000008 601.61191 601.95507
## FBgn0000014 88.44375 78.64252
## FBgn0000015 70.94939 65.04998
## FBgn0000017 2593.05424 2334.03223
## FBgn0000018 318.78628 333.01708
## trapnell_counts_C1_R3 trapnell_counts_C2_R1
## FBgn0000003 0.0000 0.00000
## FBgn0000008 540.4924 544.48136
## FBgn0000014 100.9178 89.20881
## FBgn0000015 50.4589 56.39637
## FBgn0000017 2393.8864 2614.74099
## FBgn0000018 352.2419 311.71814
## trapnell_counts_C2_R2 trapnell_counts_C2_R3
## FBgn0000003 0.00000 0.00000
## FBgn0000008 622.53341 560.62587
## FBgn0000014 127.98795 104.35007
## FBgn0000015 72.69716 74.68191
## FBgn0000017 2672.38848 2631.25864
## FBgn0000018 294.88425 352.94877
write.csv(as.data.frame(orderedRes), file="trapnell_normCounts.DESeq2.csv")
Finally, we can merge the two tables into a single one.
merged.results <- merge(normCounts, orderedRes, by="row.names")
head(merged.results)
## Row.names trapnell_counts_C1_R1 trapnell_counts_C1_R2
## 1 FBgn0000003 0.00000 0.00000
## 2 FBgn0000008 601.61191 601.95507
## 3 FBgn0000014 88.44375 78.64252
## 4 FBgn0000015 70.94939 65.04998
## 5 FBgn0000017 2593.05424 2334.03223
## 6 FBgn0000018 318.78628 333.01708
## trapnell_counts_C1_R3 trapnell_counts_C2_R1 trapnell_counts_C2_R2
## 1 0.0000 0.00000 0.00000
## 2 540.4924 544.48136 622.53341
## 3 100.9178 89.20881 127.98795
## 4 50.4589 56.39637 72.69716
## 5 2393.8864 2614.74099 2672.38848
## 6 352.2419 311.71814 294.88425
## trapnell_counts_C2_R3 baseMean log2FoldChange lfcSE stat
## 1 0.00000 0.00000 NA NA NA
## 2 560.62587 578.61667 -0.01363018 0.09778363 -0.1393912
## 3 104.35007 98.25848 0.26279172 0.22066004 1.1909348
## 4 74.68191 65.03895 0.12821306 0.26373025 0.4861523
## 5 2631.25864 2539.89350 0.11317799 0.05904858 1.9166927
## 6 352.94877 327.26607 -0.06538546 0.12276560 -0.5326041
## pvalue padj
## 1 NA NA
## 2 0.88914103 0.9992327
## 3 0.23367917 0.9515670
## 4 0.62685920 NA
## 5 0.05527698 0.6446372
## 6 0.59430768 0.9992327
Exercise: When merging the two tables, we lost the ordering by p-value. Can you reorder the merged.results table by p.value?
Click Here to see the answer
merged.results <- merged.results[ order(merged.results$padj), ] head(merged.results)
## Row.names trapnell_counts_C1_R1 trapnell_counts_C1_R2 ## 124 FBgn0000370 8501.291 8316.689 ## 3545 FBgn0030362 10506.340 10111.874 ## 13110 FBgn0086904 10371.245 10938.106 ## 9801 FBgn0039830 8337.039 8705.047 ## 2063 FBgn0022893 8526.561 8671.066 ## 14818 FBgn0263598 3419.177 3536.001 ## trapnell_counts_C1_R3 trapnell_counts_C2_R1 trapnell_counts_C2_R2 ## 124 8562.486 22004.84 22694.824 ## 3545 10902.032 26508.35 25890.427 ## 13110 10568.227 27030.27 28008.884 ## 9801 8094.771 20488.29 20395.136 ## 2063 8991.387 21115.83 22103.008 ## 14818 3565.115 8983.43 8519.902 ## trapnell_counts_C2_R3 baseMean log2FoldChange lfcSE stat ## 124 22378.998 15409.855 1.402136 0.04082003 34.34921 ## 3545 26029.204 18324.704 1.315089 0.04352489 30.21463 ## 13110 26143.785 18843.419 1.348634 0.04462244 30.22321 ## 9801 20257.213 14379.583 1.282328 0.04359018 29.41781 ## 2063 23197.430 15434.213 1.342575 0.04778029 28.09893 ## 14818 8828.834 6142.077 1.323648 0.04757299 27.82351 ## pvalue padj ## 124 1.447240e-258 9.535863e-255 ## 3545 1.521793e-200 3.342366e-197 ## 13110 1.173879e-200 3.342366e-197 ## 9801 3.250763e-190 5.354819e-187 ## 2063 1.009490e-173 1.330306e-170 ## 14818 2.253991e-170 2.475257e-167
Visualizing results
DESeq2 provides several functions to visualize the results, while additional plots can be made using the extensive R graphics capabilities. Visualization can help to better understand the results, and catch potential problems in the data and analysis. We start here by reproducing the plots that we previously obtained using Galaxy.
Dispersion plot
We can plot the DESeq2 dispersion re-estimation procedure by typing:
plotDispEsts(ddsHTSeq)
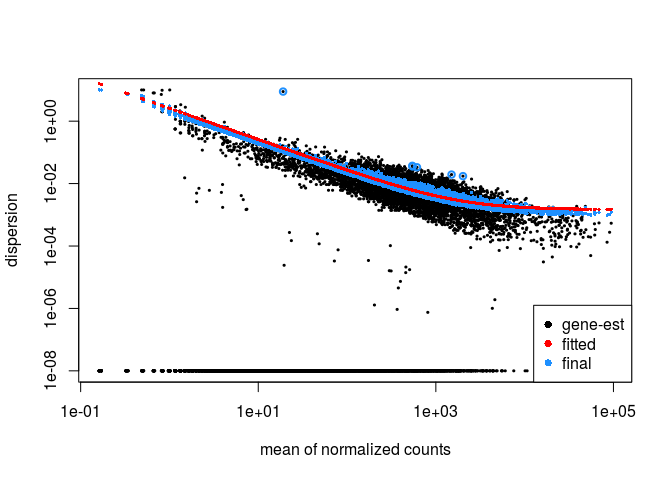
P-value distribution
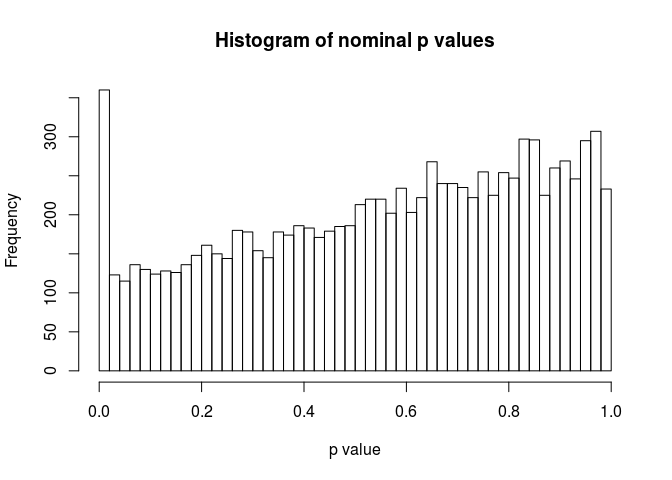
As a sanity check, we can inspect the distribution of p-values using the hist function.
hist(resHTSeq$pvalue, breaks=0:50/50, xlab="p value", main="Histogram of nominal p values")
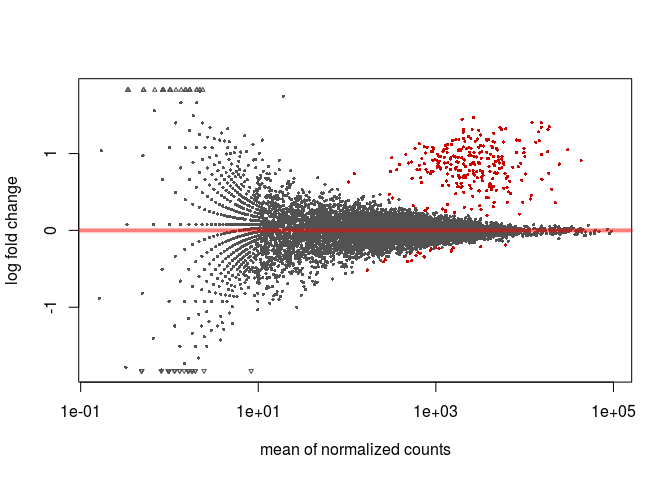
MA-plot
To make an (unshrunken) MA-plot, that displays the relationship between a genes’ mean expression and its fold-change between experimental conditions, type the following in the R console.
plotMA(resHTSeq)
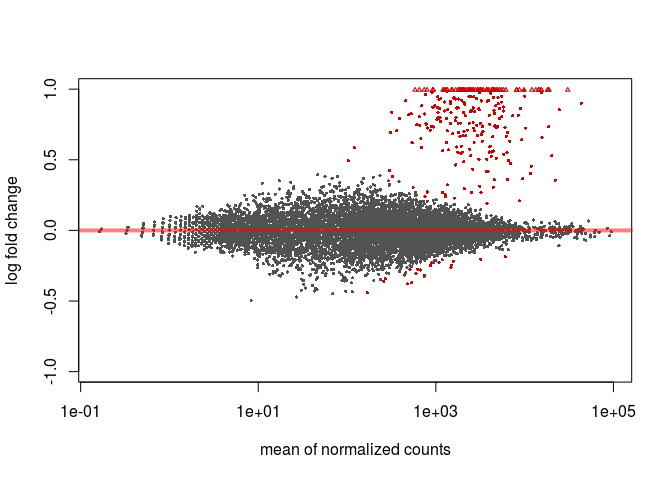
To obtain an MA-plot with shrunken log2 fold-changes we use the lfcShrink function. This function is equivalent to the results function that we called previously, but will return a table with the log2FoldChange and lfcSE columns replaced with the shrunken values. The coef argument is used to specify what contrast we are interested in analysing (in this case condition_C2_vs_C1), so we first call resultsNames to determine the right coefficient.
resultsNames(ddsHTSeq)
## [1] "Intercept" "condition_C2_vs_C1"
resHTSeqShrunk <- lfcShrink(ddsHTSeq, coef=2)
plotMA(resHTSeqShrunk)
Volcano plot
A Volcano plot displays the relationship between fold-change and evidence of differential expression (represented as -log p-adusted). DESeq2 doesn’t provide a function to display a Volcano plot, but we can create one using R’s base plot functions. In red we highlight genes differentially expressed with Padj < 0.05.
highlight <- which(resHTSeq$padj < 0.05)
plot(resHTSeq$log2FoldChange, -log10(resHTSeq$pvalue), xlab="log2 Fold-change", ylab="-log P-adjusted", pch=20, cex=0.5)
points(resHTSeq$log2FoldChange[ highlight ], -log10(resHTSeq$pvalue[ highlight ]), col="red", pch=20, cex=0.5)
abline(v=0, h=-log10(0.05), lty="dashed", col="grey")
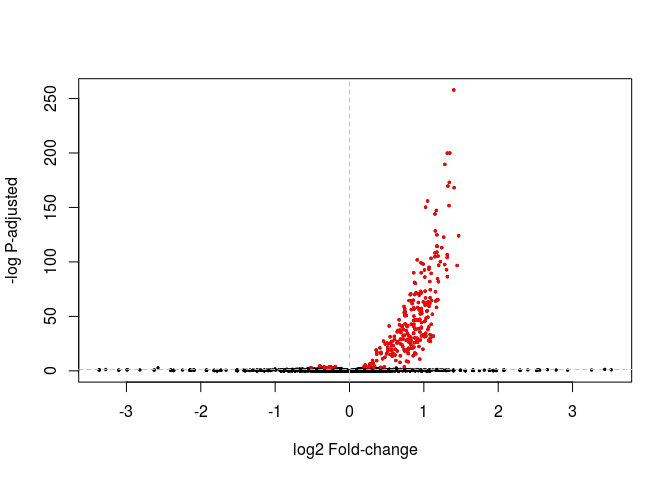
Exercise: Change the commands above to make a volcano plot using the shrunken log fold changes instead. Also change the threshold of differential expression to 0.01 and the color of the differentially expressed genes to green.
Click Here to see the answer
highlight <- which(resHTSeqShrunk$padj < 0.01) plot(resHTSeqShrunk$log2FoldChange, -log10(resHTSeqShrunk$pvalue), xlab="shrunken log2 Fold-change", ylab="-log P-adjusted", pch=20, cex=0.5) points(resHTSeqShrunk$log2FoldChange[ highlight ], -log10(resHTSeqShrunk$pvalue[ highlight ]), col="green", pch=20, cex=0.5) abline(v=0, h=-log10(0.01), lty="dashed", col="grey")
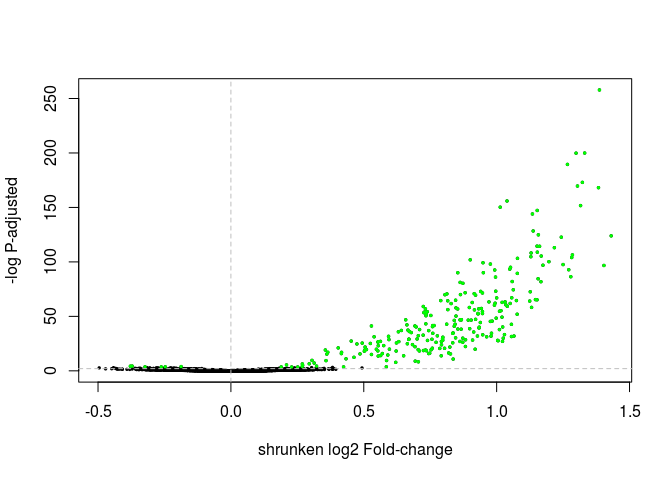
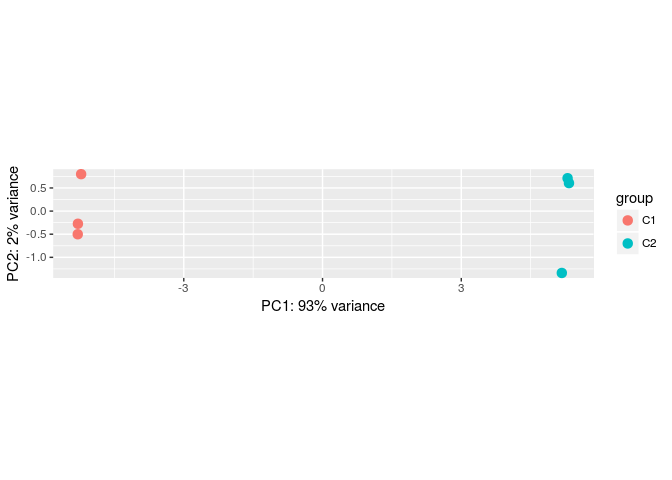
Principal component analysis (PCA)
DESeq2 provides a function to make a Principal Component Analysis (PCA) of the count data. The DESeq2 vignette recommends using transformed counts as input to the PCA routines, as these transformations remove the dependence of the sample-to-sample variance on the genes’ mean expression.
One such transformations is the variance stabilizing transformation (VST). You can type ?varianceStabilizingTransformation to learn more about this. To compare samples in an manner unbiased by prior information (i.e. the experimental condition), the blind argument is set to TRUE.
transformed.vsd <- varianceStabilizingTransformation(ddsHTSeq, blind=TRUE)
## -- note: fitType='parametric', but the dispersion trend was not well captured by the
## function: y = a/x + b, and a local regression fit was automatically substituted.
## specify fitType='local' or 'mean' to avoid this message next time.
plotPCA(transformed.vsd)
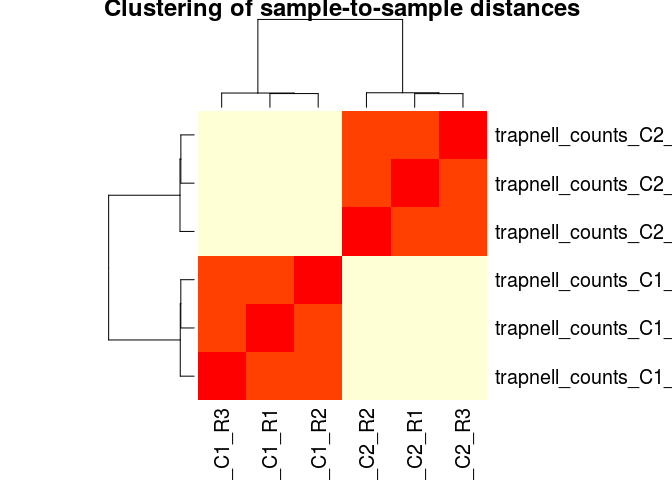
Sample-to-sample correlation heatmap
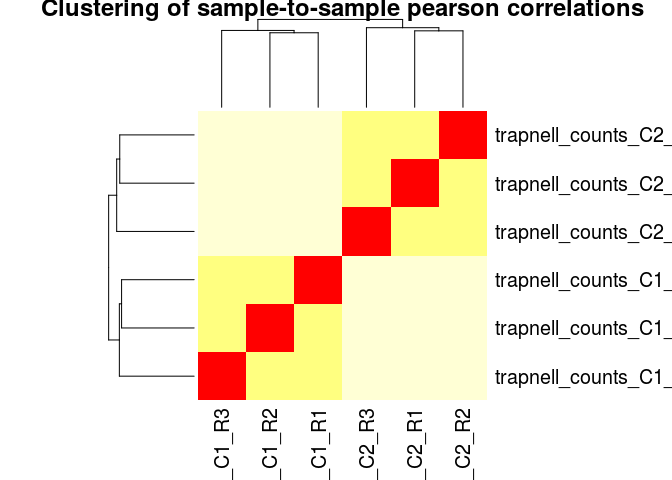
Another common visualization of high-throughput datasets is a clustered heatmap of sample-to-sample distances (or correlations). This visualization groups togheter the samples that are more similar to each other.
To make this visualization we first calculate a matrix of distances between all pairs of samples. Then we use the heatmap (from the base R package) to cluster and display the heatmap.
dists <- as.matrix(dist(t(normCounts)))
heatmap(dists, main="Clustering of sample-to-sample distances", scale="none")
We can also use pearson (or spearman) correlations as a distance metric. This is more robust than simple euclidean distances, and has the advantage that we can even use the raw (non-normalized) counts as input. It is generally a good idea to log transform the counts first.
log10_rawCounts <- log10(counts(ddsHTSeq) + 1)
dists <- 1 - cor(log10_rawCounts, method="pearson")
heatmap(dists, main="Clustering of sample-to-sample pearson correlations", scale="none")
Other visualizations
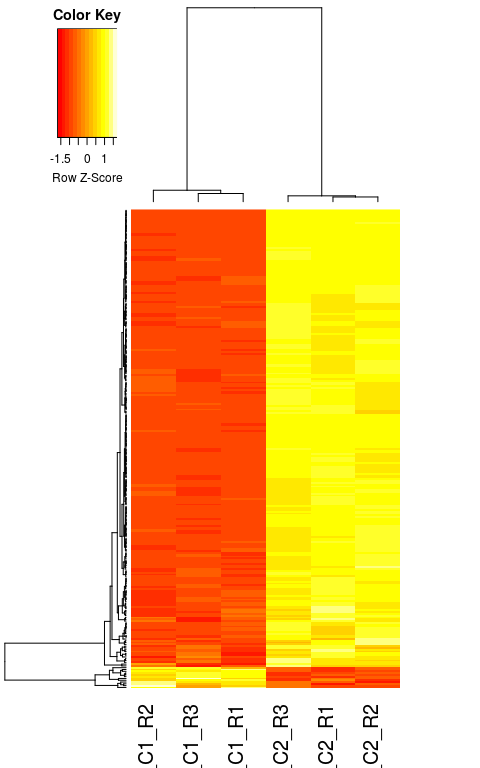
Here we plot the relative expression of all differentially expressed genes in the 6 samples. This figure is useful to visualize the differences in expression between samples.
library(gplots)
##
## Attaching package: 'gplots'
## The following object is masked from 'package:IRanges':
##
## space
## The following object is masked from 'package:S4Vectors':
##
## space
## The following object is masked from 'package:stats':
##
## lowess
diffgenes <- rownames(resHTSeq)[ which(resHTSeq$padj < 0.05) ]
diffcounts <- normCounts[ diffgenes, ]
heatmap.2(diffcounts,
labRow = "",
trace = "none", density.info = "none",
scale = "row",
distfun = function(x) as.dist(1 - cor(t(x))))
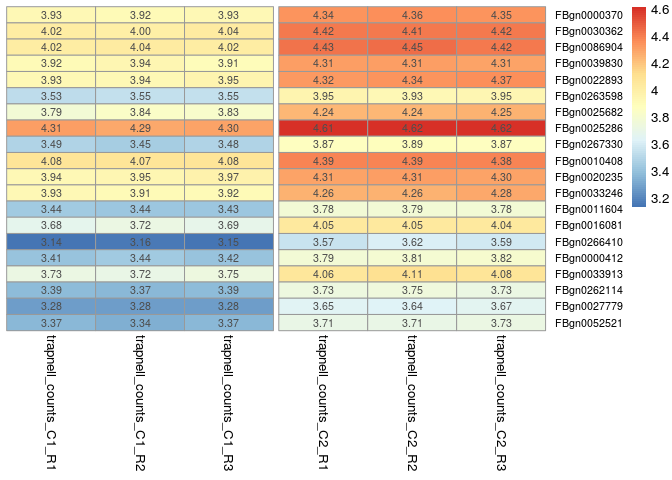
The following commands are used to plot a heatmap of the 20 most differentially expressed genes. For this, we use the ordered results table to determine which genes are most differentially expressed, and then plot the values from the normalized counts table (transformed to log10).
library(pheatmap)
# select the 20 most differentially expressed genes
select <- row.names(orderedRes[1:20, ])
# transform the counts to log10
log10_normCounts <- log10(normCounts + 1)
# get the values for the selected genes
values <- log10_normCounts[ select, ]
pheatmap(values,
scale = "none",
cluster_rows = FALSE,
cluster_cols = FALSE,
fontsize_row = 8,
annotation_names_col = FALSE,
gaps_col = c(3,6),
display_numbers = TRUE,
number_format = "%.2f",
height=12,
width=6)
Session information
sessionInfo()
## R version 3.4.4 (2018-03-15)
## Platform: x86_64-pc-linux-gnu (64-bit)
## Running under: Ubuntu 16.04.4 LTS
##
## Matrix products: default
## BLAS: /usr/lib/libblas/libblas.so.3.6.0
## LAPACK: /usr/lib/lapack/liblapack.so.3.6.0
##
## locale:
## [1] LC_CTYPE=pt_PT.UTF-8 LC_NUMERIC=C
## [3] LC_TIME=pt_PT.UTF-8 LC_COLLATE=en_US.UTF-8
## [5] LC_MONETARY=pt_PT.UTF-8 LC_MESSAGES=en_US.UTF-8
## [7] LC_PAPER=pt_PT.UTF-8 LC_NAME=C
## [9] LC_ADDRESS=C LC_TELEPHONE=C
## [11] LC_MEASUREMENT=pt_PT.UTF-8 LC_IDENTIFICATION=C
##
## attached base packages:
## [1] parallel stats4 stats graphics grDevices utils datasets
## [8] methods base
##
## other attached packages:
## [1] pheatmap_1.0.8 gplots_3.0.1
## [3] DESeq2_1.18.1 SummarizedExperiment_1.8.1
## [5] DelayedArray_0.4.1 matrixStats_0.52.2
## [7] Biobase_2.38.0 GenomicRanges_1.30.1
## [9] GenomeInfoDb_1.14.0 IRanges_2.12.0
## [11] S4Vectors_0.16.0 BiocGenerics_0.24.0
##
## loaded via a namespace (and not attached):
## [1] bit64_0.9-7 splines_3.4.4 gtools_3.5.0
## [4] Formula_1.2-2 latticeExtra_0.6-28 blob_1.1.0
## [7] GenomeInfoDbData_1.0.0 yaml_2.2.0 pillar_1.3.0
## [10] RSQLite_2.0 backports_1.1.2 lattice_0.20-35
## [13] digest_0.6.16 RColorBrewer_1.1-2 XVector_0.18.0
## [16] checkmate_1.8.5 colorspace_1.3-2 htmltools_0.3.6
## [19] Matrix_1.2-14 plyr_1.8.4 XML_3.98-1.9
## [22] genefilter_1.60.0 zlibbioc_1.24.0 xtable_1.8-2
## [25] scales_1.0.0 gdata_2.18.0 BiocParallel_1.12.0
## [28] htmlTable_1.11.1 tibble_1.4.2 annotate_1.56.1
## [31] ggplot2_2.2.1 nnet_7.3-12 lazyeval_0.2.1
## [34] survival_2.42-3 magrittr_1.5 crayon_1.3.4
## [37] memoise_1.1.0 evaluate_0.10.1 foreign_0.8-70
## [40] tools_3.4.4 data.table_1.11.4 stringr_1.3.1
## [43] munsell_0.5.0 locfit_1.5-9.1 cluster_2.0.6
## [46] AnnotationDbi_1.40.0 compiler_3.4.4 caTools_1.17.1
## [49] rlang_0.2.2 grid_3.4.4 RCurl_1.95-4.10
## [52] rstudioapi_0.7 htmlwidgets_1.2 bitops_1.0-6
## [55] base64enc_0.1-3 labeling_0.3 rmarkdown_1.9
## [58] gtable_0.2.0 DBI_0.7 gridExtra_2.3
## [61] knitr_1.20 bit_1.1-12 Hmisc_4.1-1
## [64] rprojroot_1.3-2 KernSmooth_2.23-15 stringi_1.2.4
## [67] Rcpp_0.12.18 geneplotter_1.56.0 rpart_4.1-13
## [70] acepack_1.4.1
Back
Back to previous page.